Episode 0.3: Light 101 (bonus ep.)
In this “bonus” episode, we talk about the basics of the electromagnetic and visual spectra, touch very briefly on human vision and then review Kelvin temp and Color Corrected Temperature.
SHOW NOTES, CHARTS, AND TRANSCRIPT BELOW
Source:United States Department of Energy
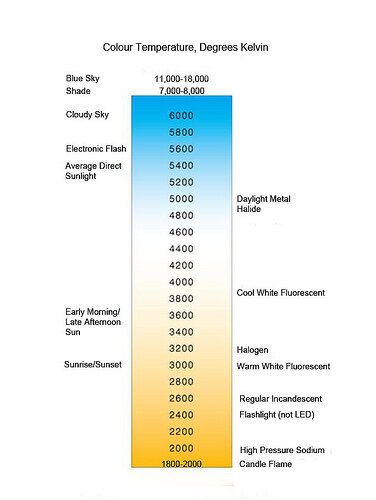
One of the better basic K-Temp charts I found; the only caveat is that it includes fluorescent lighting on here which is good for comparison but keep in mind that fluorescent and LED bulbs should be classified by their CCT.
Annoyingly I can’t track down the source even with a Google search so if anyone wants to let me know who to credit, please drop me a line.
Handy-Dandy Links As Promised
BASIC STARTING POINTS
Encyclopedia Britannica’s “Light” entry is, as one would expect, thorough and approachable:
https://www.britannica.com/science/light
Wikipedia’s pretty solid when it comes to basic physics stuff. These articles are good starting points but they can contradict each other in small ways (for instance, the exact wavelengths of specific colors) :
Electromagnetic spectrum:
https://en.wikipedia.org/wiki/Electromagnetic_spectrum
Visible spectrum
https://en.wikipedia.org/wiki/Visible_spectrum
Light
https://en.wikipedia.org/wiki/Light
Color Temperature
https://en.wikipedia.org/wiki/Color_temperature
Black Body Radiation
https://en.wikipedia.org/wiki/Black-body_radiation
FURTHER READING:
A very thorough primer about electromagnetic spectrum and radiation dosages, for the curious:
https://www.energy.gov/sites/prod/files/2018/01/f46/doe-ionizing-radiation-dose-ranges-jan-2018.pdf
For my fellow artists, here’s a great breakdown of color temperature and color theory in art, with a great explanation of the Plank curve and the relation of blackbody radiation to the CIE colorspace; by Bruce MacEvoy over at his site, Handprint:
https://www.handprint.com/HP/WCL/color12.html
VERY HELPFUL DEAD-TREE SOURCES
Architectural Lighting: Designing with Light and Space, by Herve Descottes with Cecilia E. Ramos. 2011. Princeton Architectural Press.
Color, Environment, and Human Response: An Interdisciplinary Understanding of Color and its Use as a Beneficial Element in the Design of the Architectural Environment, by Frank Mahnke. 1996. Van Nostrand Reinhold.
RANDOM RELATED READING I THOUGHT WAS AMUSING:
Part of a great Stack Exchange answer to the question “Why can’t we see infrared?”, provided by “Rob”. Quoting here at length (but there’s more at the link!) because it’s another straightforward explanation of the physics of light and how it effects the visual system, and it might land better for some:
“Light is the way that electric charges exchange energy with each other. Light comes in lumps, called "photons," which each carry a certain amount of energy. It turns out that the energy in each lump is directly related to its color: violet light has more energy per lump than blue, blue more than green, green more than yellow, yellow more than red, and red more than infrared. When visible light hits the pigment proteins in the retina, it makes the electrons vibrate; that sets in motion the machinery to send an electrical impulse to your brain. When ultraviolet light hits those pigment molecules it ionizes them, which makes the molecules fall apart and sets in motion a different mechanism ("cleanup on aisle four"). And when infrared light hits those pigment molecules, it doesn't have enough energy to make the electronic vibrations go, so you get zero information about the infrared light: you're at the vending machine [that takes only bills and quarters], but only with dimes. Visible light photons have energies from about 1.8 volts (red) to about 3 volts (violet).
The whole story is more complicated than this because the different ways a molecule can vibrate depend very sensitively on its shape, but that's the basic idea.”
https://physics.stackexchange.com/questions/209854/why-cant-we-see-infrared-light
FULL SHOW TRANSCRIPT
Welcome to Episode 0.3 of Starlight and Fireflies, a podcast dedicated to understanding and eliminating the harmful effects of artificial light at night.
This is a “bonus” episode covering the basic science of light - the electromagnetic spectrum, including the visual spectrum of course, high vs.low energy wavelengths, touching on human light perception and finally the critical concepts of K temp and CCT, or “correlated color temperature”.
I decided to do this Light 101 because I’ve found that it’s useful for about one third to one half of the people I’ve taught in classes and lectures. If any of these things are old hat for you, feel free to skip this one and head over to Episode 1.0 - Blue Light Special #1
OK, first thing’s first: I’m gonna admit right now, it’s tricky to talk about an inherently visual phenomenon in a strictly audio format, usually I have slides, but we’ll get through this together. I’ve got a couple of charts and some handy-dandy links available in the show notes on starlightandfireflies.com; and a full transcript as always. Also: I am not a physicist, I don’t even play one on TV, I’m fairly confident in my explanations but if I’ve bungled something horribly please drop me a line at podcast@starlightandfireflies.com and let me know!
SO: let’s start at the beginning.
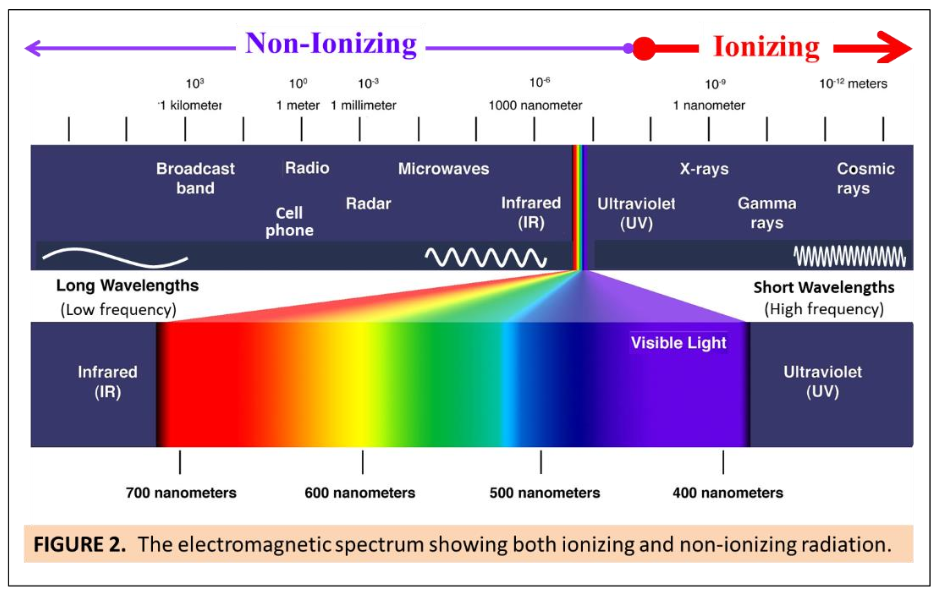
All light is, is radiation - it is the visible part of the electromagnetic spectrum. Everything gives off radiation of some type, no matter how low energy or how undetectable by our relatively limited human senses. Case in point - humans emit electromagnetic waves in the infrared band all the time! We can’t see it, we just call it “body heat”.
The three properties of electromagnetic radiation are wavelength, frequency and energy; frequency and energy are proportional, wavelength and frequency are inverse, meaning the longer the wavelength the slower and lower energy it is, and the shorter the wavelength the faster and higher energy that is.
If it just sounded like I talked in circles, let’s deconstruct:
Think about any charts or pictures you’ve seen of radio waves or an oscilloscope screen - the wavy line with peaks and valleys, with a line through the middle of it.
The distance between the center of one peak and another peak, or one valley and another valley, is called the wavelength. Wavelengths can be (theoretically) as long as the universe or as short as sub-atomic, regardless of length they are measured in meters whether kilometers or nanometers.
The distance between the straight line and the top or bottom of the wave is called the amplitude of a wave, I like to think of it as basically how *much* of the wavelength there is. A long wave and a short wave at the same amplitude will carry different amounts of energy, with the short wave being more energetic because it has a much higher frequency.
And the frequency of a wave is measured in Hertz, and is defined by the number of times a peak of a wave passes a given point in a second, and is directly proportional to the energy it carries. A long wavelength is going to take a longer time to get from peak to peak, so it’s slower and carries less energy than a shorter wavelength of the same height. So, again, the shorter the wave, the faster it travels and the more energy it has.
People often use frequency and wavelength interchangeably when they’re talking about the energy potential of a given radiation wave. Which makes sense, but it’s an inverse relationship that can get confusing. High frequency = short wavelength, and visa versa.
To ground this a bit:
Radio waves are huge and move relatively slowly (like, down to 3 Hz for some radiowaves we use) and pass through mountains. Gamma rays scream through the universe at 300 exahertz and can shear particles off atoms. (So 300 “exahertz” means the wave is moving at 300 times 10-with-18-zeros PER SECOND, which is a number I’m not ashamed to admit breaks my brain a little). A radio wave at the same amplitude as a gamma ray is exponentially longer and slower than the gamma.
It’s the difference between a faucet pouring water over your hands (low frequency/low energy) and a water jet cutter (extremely high frequency/highenergy).
The faucet will wash your hands off.
The waterjet will CUT your hands off.
The important thing to remember is that shorter wavelengths = more energy.
Because they behave so differently, the waves at the higher and lower ends of the spectrum interact with matter very differently. The waves at the high end of the energy spectrum - some types of UV, X-rays, gamma, and so forth are called “ionizing radiation”, because they carry enough energy to actually knock electrons off of atoms, ionizing them, which can eventually change the behavior of the molecule.
From lower-energy UV on down through radio waves including the visual spectrum, the wavelengths have enough energy to excite the molecules they come into contact with, but can’t break the valence bonds holding the electrons, so they are called non-ionizing.
This does not mean they have NO EFFECT. That excitation of molecules is, after all, what cooks food in a microwave - the waves are long enough to penetrate the surface of the food and not just irradiate the surface like the infrared of regular ovens, so they transfer enough energy to the molecules within the food that they vibrate, heating them up, without ionizing. (But still sometimes burning).
And this excitation of energy is exactly what makes it possible for us to SEE. I’ll tackle the visual system in much more depth in another episode, but the simplest breakdown is this:
We perceive colors and light and dark, and light is a major driver of our circadian rhythms, is because the chemicals in our cones and rods and the intrinsically photosensitive retinal ganglion cells each react to *specific* electromagnetic wavelengths in the visual spectrum. When certain wavelengths hit the photoreactive enzyme which reacts to it, it sets off this whole complex set of biological and neurochemical chain reactions that at the end of it is “sight”.
Humans eyes evolved to see wavelengths of light approximately 380-740 nanometers long. It’s not a very wide range, but it’s what we’ve got, and that’s the “visual spectrum”.
So picture a rainbow in your head. The violet on one side of that rainbow is at 380-ish nanometers, and going back through the rainbow you’ve got blue, cyan, green, yellow, orange, and finally red at approx 740.
The upper and lower limits of the visual spectrum are pretty well defined - which is why red and violet have numbers with them - but the exact colors and their wavelength equivalents are absolutely not well defined, to the tune of I have looked at many different sources and and none of them agree on which wavelengths are which colors. I’ll talk about that more in Episode 1 when we try to pin down a set definition of “blue light”.
Putting the visual spectrum in context of the rest of the electromagnetic spectrum - outside of the rainbow, just past violet are the UVwaves, which we can’t see but lots of other creatures can. On the other side of the rainbow, outside of red, there’s the infra-red waves and far infra-red. We can’t see those either.
But we experience the UV and IR every day -the entire UV to rainbow to IR spectrum exists in sunlight (the sun gives off plenty of other radiation, too, but the atmosphere blocks a whole bunch of it). We experience IR as the heat of the sun and UV as the sunburn we get if we indulge in it too long for our skin type.
Part of the reason we don’t see in UV and IR is because it didn’t give us enough of an evolutionary advantage to do so.
UV does pack enough energy to break things, and the damage is cumulative over time, so our eyes evolved corneas and other layers to block a lot of it out in order to save the more delicate parts of our visual systems (like lenses and retinas) but enough damage can still accumulate over the course of our long lifespans that we suffer things like cataracts and macular degeneration. Interestingly enough, we DO see some of the longer UV wavelengths which are between 320-400 nm and they overlap with the violet, which starts at 380.
This is why it’s critical to wear uv-blocking eyes protection when you’re outdoors, and why we should be a little wary of white-light-emitting devices (like streetlights) if we don’t know exactly what wavelengths they are emitting.
And if not for us, than for the creatures who CAN see more of the UV range. Creatures who don’t live as long are sometimes able to see deeper into the violet ranges, including UV, since their eyes didn’t evolve to block out the cell damaging rays. Some insects and some birds, for example - with shorter life spans, they don’t accumulate enough damage to their eyes through UV exposure to go blind before they die - so it makes sense that they would use those abilities to their advantage in other ways, such as UV-reflecting plumage or being able to see which stamen haven’t been visited yet by other bees, because the UV-reflecting pollen glows just a little bit brighter.
The downside of those abilities, now, is that we use lights outside that sometimes throw off lightwaves in the deep violet and UV range - we might remain completely oblivious, while some insects might see it and change their behavior as a result.
I haven’t been able to find a similar evolutionary reason why mammals, including us, don’t really see in IR unless the source is exceptionally strong (like, laser-beam strong), so if any of you have information or ideas as to why that is, drop it on me, I’m really curios! Email it to podcast@starlightandfireflies.com
And that’s as deep as I’m gonna go into the human visual system and the electromagnetic and visual spectra for this episode - there’s lots to talk about regarding the amazing process that is our vision, so we’ll deal with that in at least one other separate episode.
The final things I want to cover, now, are the related concepts of K-temp and Correlated Color Temperature. This is a critical aspect of lighting and electric light generation and I will talk about K-temp and CCT a lot, but I pretty much almost always say “Ktemp” since CCT is generally reported in degrees Kelvin anyway.
K-temp is short for Kelvin temperature, the unit of measurement of the thermodynamic temperature scale... It’s a scale anchored in absolute zero, which is the theoretical temperature at which all atomic motion ceases. (It’s theoretical because it’s never been reached, but scientists have come close.)
Real talk, here: There is a lot of complicated math and physics around K temp (things like CIE color space and Planck’s law and black body radiation curves show up) but here’s the basics that we need to know about for OUR purposes when we’re talking about not hurting ourselves or animals with artificial light at night:
So, here goes: when anything burns - basically, when the atoms are excited - it releases energy in the form of radiation. At lower temperatures, it releases infrared - the stuff we can’t see, but instead sense as heat. As the temperature increases, so does the energy level of the radiation emitted by the object. So as it gets hotter, it will emit wavelengths in the visual spectrum, starting with a deep red glow, then yellow, then white; if it keeps getting hotter still it will emit a pale blue light then a deep brilliant blue. Heat it up even more - or excite the electrons even more - and you start getting further into the UV and xrays and other ionizing radiation.
This red-to-yellow-to-white-to-blue shift is a predictable, measurable thing when elements burn, and because of this constant we’ve been able to extrapolate the size, temperature, and elemental composition of stars, including our sun.
Putting K-temp in regular-object context:
At the redder but cooler part of the spectrum: A candle burns at around 1700 degrees K; the old fashioned sodium streetlights are around 2000k; basic incandescent light bulbs are between 2000 and 2800K. Halogens are between approximately 3200 and 5ooo depending on the halide used. At the blue-but-hotter side, the sun is around 5200-5800K; overcast days can be anywhere from 6000k to 10,000 K.
It’s one of the many places where our cultural definitions and human perceptions go against what the physics tell us - the lower the K-temp, the redder the light source. The higher the k-temp, the whiter/bluer the light source.
K-temp is a set and known thing when you’re talking about natural or incandescent light sources, because the light they throw off is in a set and smooth curve.
Sunlight contains, as one would expect, high amounts of all visual wavelengths, with variations according to what the rest of the atmosphere is doing - for instance, the light of an overcast but bright day looks completely different - and much cooler - than the golden light of a sunset.
Incandescent sources - firelight, our old light bulbs - also throw off a continuous, smooth curve of light, kind of a ski slope through the visual spectrum with the low end in the violets and the highest part in the oranges and reds.
But electro phosphorescent light sources like LEDs and fluorescents excite the electrons of various materials until they throw off light, but they don’t emit the smooth spectral curves of wavelengths the way the incandescents do. We’ll get into the why and hows and more details when we discuss the vagaries of the current light bulb aisle, but basically the “K-temp” you see on packages for LEDs is NOT actually K-temp. It’s called CCT.
So “CCT” stands for “Correlated Color Temperature” – and it’s called ‘correlated’ because CCT is a calculated value. It’s figured out by mapping a light’s perceived whiteness and color value to it’s closest approximate K-temperature.
It is a standardized equation. However, that standardization does not always translate into uniformity in the real world. Two lamps may claim to have the same CCT value, but will seem to be glaringly different, because the materials used to create that K-temp are different depending on manufacturer or even batch!
More frustratingly, even when LEDs are labeled as having a CCT of 2700 k - which, in an incandescent, there would be very little high-energy wavelengths being emitted at all - there can be hidden spikes of blue, green, or violet lightwaves coming from the LED, depending on how the bulb was made, and as a consumer standing in a hardware store we can’t really know.
The reason all of this is important to know is because artificial light at night has radically transformed in just 20 years, from a primarily low-K-temp environment to one increasingly dominated by high-K-temp sources - lights that are emitting higher-energy wavelengths.
Humans, animals, and plants are used to timing a lot of important biological functions based on when they are exposed to different k-temps of light - the 2700K light of a red sunset, or the approximate 4100K silver of moonlight, which is a monthly thing and not an every-day thing. Now there’s suddenly blue-white “daylight” streetlights outside and in-home lighting for use after dark - and it’s demonstrably Not Good, and we’ll get into what and why over the course of this entire podcast.
Starting with the next episode “Blue Light Special #1”, we’re going to try to nail down what “blue light” is, exactly, specifically, and talk about why knowing the wavelengths that are emitted by the things around us matter, using a rather dramatic example.
Until next time, if you get a chance to see the night sky, take it, because that starlight is your birthright.